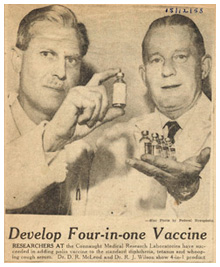
Other Vaccines
In addition to the four main vaccines featured on this site and in the original exhibit, there are several others with significant Canadian elements in the history of their development and/or application. They include vaccines against rabies, tuberculosis, tetanus, influenza, measles and haemophilus influenza type b, or simply Hib.
Rabies

Image source: Wikipedia/ CDC (public domain image)
Rabies is an acute, progressive viral disease of the central nervous system transmitted from animal to animal, or animal to human, via exposure to saliva. Derived from the Latin word for “madness,” and characterized by hydrophobia, or “fear of water,” rabies is most often spread through animal bites and is virtually 100% fatal unless treated with rabies vaccine. After a rabid bite, the virus attaches to nerve endings and then slowly and inexorably travels to the brain.
“I have seen agony in death only once, in a patient with rabies; he remained acutely aware of every stage in the process of his own disintegration over a twenty-four-hour period, right up to his final moment” (Lewis Thomas, The Lives of a Cell, New York: 1974).
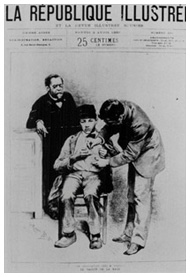
 Louis Pasteur (left) and Joseph Meister receiving the first Pasteur Rabies Treatment, 1885.
Louis Pasteur (left) and Joseph Meister receiving the first Pasteur Rabies Treatment, 1885.Image source: Sanofi Pasteur Canada
Rabies is an ancient disease, indeed perhaps the oldest infectious disease known, and was widespread in the Old World. However, rabies was not present among New World terrestrial mammals until the arrival of Europeans in the 16th century, although it was present among some flying mammals, such as vampire bats. The disease soon became established in several animals, particularly dogs, and remained a much feared disease threat until Louis Pasteur’s successful development of the Pasteur Rabies Treatment in 1885.
During the 1870s, after developing vaccines against cholera, anthrax and swine fever in livestock, Pasteur turned his attention to rabies in his Paris lab. He was soon able to culture an invisible microorganism in rabbit spinal cord, which later proved to be the rabies virus. He then weakened its virulence after exposing the infected rabbit spinal cord to dry air for a period of time. The infected cord material was then prepared into a vaccine. After encouraging tests on dogs exposed to the virulent rabies virus, Pasteur made several initial human tests of his vaccine. He was unsuccessful until July 8, 1885, when Joseph Meister, a teenage boy who had been bitten 14 times by a rabid dog, was given a series of inoculations over 10 days and survived. Pasteur's successes were widely celebrated, prompting the creation of the Pasteur Institute in Paris in 1888 to further expand vaccine production and research.
 William Fenton, Canada’s Joseph Meister, Ontario Provincial Laboratories, 1913.
William Fenton, Canada’s Joseph Meister, Ontario Provincial Laboratories, 1913.Image source: Sanofi Pasteur Canada
Rabies outbreaks among dogs occurred periodically in Canada during the late 1800s and early 1900s leading to humans being bitten and infected and some dying of rabies when the Pasteur Rabies Treatment could not be given in time, or was unavailable. Prior to 1913, Canadian victims of rabid animal bites had to travel to Pasteur Institutes in the U.S., particularly in New York City, to receive treatment, although it was sometimes shipped across the border. A serious rabies outbreak in southern Ontario in 1910 prompted calls for the Pasteur Rabies Treatment to be prepared in Canada.
Dr. John G. FitzGerald made the first rabies vaccine in Canada at the Ontario Provincial Laboratory in 1913 and it was then prepared at Connaught Laboratories. FitzGerald learned how to prepare this life-saving vaccine at the Pasteur Institute in Brussels in 1910 and taught William Fenton at the Provincial Labs. Fenton volunteered to receive the full course of treatments to test its safety and was thus described as “The Canadian Joseph Meister.”
Fortunately, cases of rabies in humans remained quite rare in Canada, but the potential for cases grew as rabies outbreaks occurred among animals, especially domestic pets, wild animals and livestock, prompting greater focus on the development and use of preventive rabies vaccines for animals. A serious rabies outbreak that began among working dogs in the North West Territories and Alberta in the late 1940s, eventually spread to southern Ontario by the early 1950s, prompting urgent efforts at Connaught to prepare rabies vaccine to protect domestic dogs, coupled with a broad program to immunize dogs and cats.
Rabies remained a significant threat during the 1960s, especially spreading from wild to domestic animals, and also a threat to people who work closely with animals. Connaught introduced a new generation of rabies vaccines for domestic and wild animal use, as well as a vaccine to prevent the disease in humans.
Tetanus
Tetanus stands apart from other vaccine preventable diseases since it is not communicable between people. Derived from the Greek terms meaning “taut” and “to stretch” that describe the prolonged contraction of muscle fibres, tetanus is caused by the bacterium, clostridium tetani, which is widespread in the environment and spread by many animals that harbor and excrete it. The disease develops when spores from the bacterium are introduced into the anaerobic (absence of oxygen) conditions found in dead or damaged cells, or deep puncture wounds, such as stepping on a rusty nail. The spores then germinate in such tissues into vegetative bacilli that release a toxin. It is tetanus toxin, spreading into the nervous system, that causes the disease, the most notable effect of which is “lock jaw.”
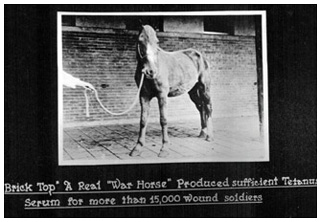
 Horses were essential for the production of tetanus antitoxin, but they were not harmed. At Connaught Labs, “Brick Top” alone supplied antitoxin for 15,000 soldiers during WWI.
Horses were essential for the production of tetanus antitoxin, but they were not harmed. At Connaught Labs, “Brick Top” alone supplied antitoxin for 15,000 soldiers during WWI.Image source: Sanofi Pasteur Canada
The cause of tetanus was not known until 1884 and the first isolation of tetanus toxin followed in 1890. Similar to diphtheria antitoxin, it was found that tetanus toxin could stimulate an immune response in large animals, particularly horses, and that their white blood cells contained antibodies. The white blood cells could then be processed into an antitoxin, which proved to be an effective treatment for people stricken with tetanus. Demand for tetanus antitoxin was limited until the outbreak of World War I, when trench warfare provided ideal conditions for wounded soldiers to be exposed to the tetanus bacteria.
A severe shortage of tetanus antitoxin for the Canadian military prompted the expansion of the Antitoxin Laboratory of the University of Toronto, leading to the establishment of a farm property in north Toronto to provide suitable space for horses and new lab facilities. The expanded operations were ready in 1916, but were not officially opened as 'Connaught Antitoxin and University Farm' until October 1917.
However, tetanus antitoxin only provided temporary immunity to the disease, prompting research along similar lines that had resulted in the development of diphtheria toxoid in the the early 1920s at the Pasteur Institute in Paris. Not long after the first paper was published in 1927 about the immunizing value of tetanus toxoid, work began at Connaught to prepare it and conduct clinical trials. By the time World War II began in 1939, Connaught was ready to supply the toxoid to the Canadian military so that soldiers would be protected. There were improvements in the production of the tetanus toxoid during the war period, and it was also combined with typhoid-paratyphoid vaccines as TABT for use in military personnel. After the war, attention focused on adding tetanus toxoid to the combined diphtheria-pertussis vaccine to create DPT, which came into wide use in Canada in 1947.
Tuberculosis
 BCG Vaccine to prevent Tuberculosis, Aventis Pasteur Canada, 2002.
BCG Vaccine to prevent Tuberculosis, Aventis Pasteur Canada, 2002.Image source: Sanofi Pasteur Canada Archives.
The complex challenge and long history of tuberculosis is the focus of an online exhibit by the Museum of Health Care entitled, “Fighting For Breath: Stopping the TB Epidemic.”
A key figure in the Canadian tuberculosis vaccine story not discussed in the online exhibit is Dr. Armand Frappier (1904-1991). Dr. Frappier established the independent, non-profit Institut de microbiologie et d’hygiène at the Université de Montréal in 1938, initially to produce the BCG tuberculosis vaccine in Canada. The Institut, later known as Institut Armand-Frappier, would also produce several other vaccines, such as diphtheria, pertussis, polio and influenza vaccines.
Frappier’s interest in the tuberculosis problem stemmed from the death of his grandmother, mother and brother due this disease. In 1931-32, after earning medical degrees from the Université de Montréal, Frappier studied tuberculosis and the BCG vaccine at the Pasteur Institute lab in Paris where it was discovered a decade earlier by Albert Calmette and Camile Guérin. Frappier was convinced of the safety and effectiveness of BCG vaccine and brought samples of the BCG strain back to Montreal, along with the know-how to prepare the vaccine.
In 1933, Frappier became professor of bacteriology at the Université de Montréal and was asked by the National Research Council of Canada to undertake a study to confirm the effectiveness of the BCG vaccine and develop methods to prepare it. Early use of BCG vaccine was focused primarily in young children in Quebec and Saskatchewan who were born into environments were TB was common, particularly aboriginal communities where the tuberculosis death rate was 10 times higher than in the non-aboriginal population. In 1930 there were 3,350 deaths from tuberculosis in Quebec alone, but there were few sanatoria in the province and the public campaign against TB was not as well developed as in other provinces.
In 1935, Frappier helped establish a BCG clinic in Montreal designed to vaccinate the newborn children of TB families. BCG proved effective in stimulating tuberculosis immunity if infants were kept isolated until a tuberculin test became positive. During this same period, Dr. R.G. Ferguson led a study focused on administering BCG among the aboriginal population of Qu’Apelle, Saskatchewan, which demonstrated a 75% reduction in mortality among BCG vaccinated children growing up in a TB environment.
In 1947, Connaught Laboratories also began to produce BCG vaccine, which was primarily provided in Ontario to individuals at special risk of tuberculosis exposure, such as student nurses, medical students and family contacts of TB cases. In Quebec, however, a broader approach was soon taken through school immunization programs. By the end of the 1950s, however, most BCG immunization programs in Canada ended following the introduction of antibiotic treatment and a rapid decline in TB incidence. BCG vaccine is still available in Canada for certain groups who are more likely to encounter the disease.
Watch the video dedicated to Dr. Armand Frappier for his induction into the Canadian Medical Hall of Fame in 2012.
Influenza
 Alberta Provincial Board of Health public health poster during influenza pandemic, 1918
Alberta Provincial Board of Health public health poster during influenza pandemic, 1918Image source: Glenbow Museum, Calgary, NA-4548-5 (public domain image)
Influenza is an acute and highly contagious viral infection caused by the influenza virus, characterized by a sudden onset of high fever, cough (usually dry), headache, muscle and joint pain, severe malaise, sore throat and runny nose. Influenza viruses spread through infected droplets and circulate in every part of the world. There are three types of influenza – A, B and C, which are further classified into, for example, Influenza A (H1N1) or A (H3N2) according the kinds and combinations of virus surface proteins. These surface proteins stimulate the production of human neutralising antibodies, which target the influenza virus and inhibit fusion of its surface proteins with cells in the upper respiratory tract, thus preventing infection. Most healthy adults and children recover without significant complications. However, the vulnerable populations, such as seniors and people with chronic medical conditions, influenza can lead to serious complications and even death.
Influenza pandemics occur when novel strains of the virus emerge among susceptible animals, especially pigs (swine) and birds, which can spread to, and then between, humans who have no immunity, younger to middle age groups being most vulnerable to the worst effects of the disease. The most recent influenza pandemic spread around the world in 2009 and involved a new strain of H1N1.
 Preparing influenza vaccine at Connaught Laboratories in 1957. The vaccine is prepared from virus cultivated in hen’s egg embryos.
Preparing influenza vaccine at Connaught Laboratories in 1957. The vaccine is prepared from virus cultivated in hen’s egg embryos.Image source: Sanofi Pasteur Canada
Almost a century ago, influenza spread around the world in an unprecedented pandemic, emerging first in China in early 1918 and finally ending a year later, the so-called “Spanish Flu” leaving far more dead in its wake than all of World War I. By the time the crisis eased, at least one sixth of the Canadian population, predominately young adults in their prime, was attacked by the flu, with some 50,000 deaths.
The “Spanish” flu pandemic had little to do with Spain except that the first news of the growing crisis originated in Spain, which was not involved in World War I and had an uncensored press. This new influenza virus strain likely first spread to France through a group of Chinese workers and then into other parts of Europe and beyond, including a U.S. military camp in Kansas, which served as a major focus of spread in North America. Ships sailing from England into Grosse Isle, Montreal and Halifax were the main routes of infection into Canada. With little understanding of the actual viral cause of the disease – the influenza virus was not isolated until 1933 - there was very little that could be done to prevent, control or treat the disease.
 Seasonal influenza vaccine prepared by Parke, Davis & Co., for the 1968-69 flu season.
Seasonal influenza vaccine prepared by Parke, Davis & Co., for the 1968-69 flu season.Museum of Health Care, accession #000001283
Nevertheless, Connaught Laboratories of the University of Toronto, launched an intensive effort to prepare a vaccine, based on suspicion of a new strain of Bacillus influenza causing the disease. Within a month, Connaught was able to distribute influenza vaccine in large quantities free of charge to provincial health departments, hospital, medical and nursing staff, the military and other public health services across the country. Due to this unprecedented emergency, no claims for the effectiveness of the vaccine were made, but it did no apparent harm and the Lab’s efforts were widely appreciated.
Influenza virus was first isolated in 1933 and could be cultivated in chicken eggs. Canadian influenza research and vaccine development began at Connaught Labs in 1936 and intensified in 1939 after the start of World War II. In 1943, the Canadian government asked Connaught to prepare vaccine for the armed forces, the effort expanding in 1944 and yielding 200,000 doses by March 1945.

Image source: Sanofi Pasteur Canada
First emerging in China in late 1956, a new pandemic strain of influenza virus (A/Asia/57) spread from Asia into Europe and North America and prompted an urgent request from the Canadian government for Connaught to supply 500,000 doses of influenza vaccine. The Institut de microbiologie et d’hygiène at the Université de Montréal was also asked to prepare vaccine. The limited supply that could be produced would be used on a priority basis, especially to protect armed forces and health services personnel. However, as vaccine became available, demand declined as the pandemic threat subsided.
In early 1976, the death of soldier in a U.S. military base from influenza identified as the same strain thought responsible for the 1918 pandemic, prompted the unprecedented effort by the U.S. government to vaccinate the entire American population. The Canadian government felt compelled to follow suit and turned to Connaught for vaccine. The ‘know how’ was available, but there was not enough time or equipment for the Labs to prepare the requested 12 million doses in time, so it imported concentrated bulk vaccine from Australia, Germany, Holland and the United Kingdom and then processed it into finished vaccine. Fortunately, the pandemic never developed, but the potential for other influenza pandemics persist, and was most recently experienced globally in 2009.
Measles
Measles is a highly contagious viral disease spread via infectious droplets through such contacts as kissing, touching, holding hands or sharing glassware or utensils, or through aerosol transmission of coughs and sneezes. The disease damages many parts of the body such as the cells lining the nose and throat, and it can weaken the immune system for months, often leading to ear infections and pneumonia. Inflammation of the brain (encephalitis) also occurs in about 1 out of every 1000 cases, which can result in brain damage. In rare cases, measles can trigger the development of a fatal brain infection that develops years after the original attack. Globally, more than 20 million people are affected by measles each year and in many developing countries, particularly parts of Asia and Africa, measles remains one of the major causes of deaths among children under the age of five.
Before the first measles vaccines were introduced in the 1960s, epidemics occurred in Canada every 2 to 3 years, with incidence peaking during the early 1950s with 61,370 cases each year at rate of 369.1 cases per 100,000 people. During this period there were also some 30 to 70 deaths each year due to measles, 5000 hospital admissions and about 400 cases of encephalitis.
The first live attenuated measles vaccine was introduced in 1963, but was not widely used in Canada until the late 1960s. In the interim, Connaught Laboratories introduced an inactivated measles vaccine that was available for infants until 1970 in an unique “Quint” combination form, DPT-Polio-Measles, which simultaneously protected against diphtheria, pertussis, tetanus, polio and measles. Following the introduction of a more effective live measles vaccine in the late 1960s, a vaccine to prevent mumps was introduced in 1967, and then a vaccine against rubella (a.k.a. German measles) in 1969. By the mid-1970s, measles vaccine was most often administered in a trivalent combination MMR form that also immunized against mumps and rubella in a single shot.
The number of measles cases in Canada has since fallen by over 99%, although in 1995 the “two dose immunization regime” was introduced as a result of 362 cases of measles being reported in Canada. All provinces and territories thus launched "catch-up" vaccination campaigns that involved some 2.1 million school children between 4 and 19 years of age being given a second booster dose of MMR in school clinics.
Measles outbreaks still occur in North America and Europe, mainly through importation, as seen quite recently in many areas, and infectious spread among un-immunized or under-immunized individuals or groups. In recent years, measles immunization rates have declines due to some parents refusing the MMR vaccine because of perceived, though quite erroneous, fears of a link between measles vaccine and the development of autism in children. Amplified by the internet, fears of such a causal link originated in a small study published in a 1998 medical journal article by Dr. Andrew Wakefield. However, by 2010, the basis of his study and his reports had been fully discredited.
Haemophilus Influenzae Type b (Hib)
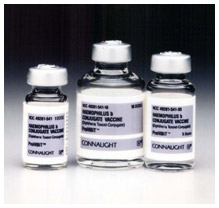
 Haemophilus influenza type b (Hib) conjugate vaccine, Connaught Laboratories, 1988
Haemophilus influenza type b (Hib) conjugate vaccine, Connaught Laboratories, 1988Image source: Sanofi Pasteur Canada
Haemophilus influenza type b (Hib) was previously known as Bacillus influenza and thought to be the cause of influenza prior to the isolation of the influenza virus in 1933. Though first identified in 1892, the particular types of H. influenzae bacteria were not differentiated until 1930. Type b is especially dangerous to infants and young children and a cause of bacteremia, pneumonia, epiglottitis and acute bacterial meningitis, and can sometimes cause cellulitis, osteomyelitis and infectious arthritis.
Hib was not a reportable disease in Canada until 1979, and only Hib meningitis was reported until 1985, at which time all invasive disease caused by Hib became reportable. However, Hib had been reportable in the United States prior to 1979 where it was clear that the disease affected children under 5 years of age, but was most serious to children 12 months and younger. Hib incidence was not especially high, but its impact was often long term, causing blindness, deafness, learning disabilities, mental health defects and physical handicaps, and it could also be fatal.
In 1988, Jessica, the nine-year-old daughter of Dr. Rob Van Exan, a key figure in the development of a new type of Hib vaccine at Connaught Laboratories, gave a speech to her class about Hib: “It causes your brain to swell, and that can cause blindness, deafness, mental retardation or death. Hib can also cause an infection of the lungs called pneumonia, or the throat or the bones or the joints. Hib is very sad because it mostly attacks children under two.”
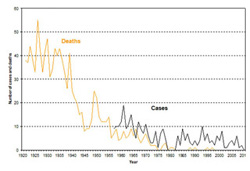
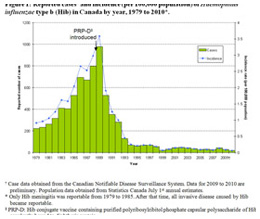
 Haemophilus influenza type b incidence, Canada, 1979-2010
Haemophilus influenza type b incidence, Canada, 1979-2010Image source: Public Health Agency of Canada
Four years earlier, her father began working with colleagues on a Hib vaccine for protecting young children most at risk. The first generation of Hib vaccine was launched in 1986, but it was minimally effective for children under two. This was because, as Jessica put it, “The immune system is something in your body that protects you from diseases, but it needs to be turned on by something like a vaccine. In young children, this system is hard to turn on.”
The new type of Hib vaccine, known as ProHIBiT®, was one of the first conjugate vaccines, incorporating key elements of diphtheria or tetanus toxoid to, in effect, “turn on the immune system even in young babies,” as Jessica described it. “Because the new vaccine works in children under two, it can stop most of the (Hib) disease.” Indeed, since the introduction of the Hib conjugate vaccine in the late 1980s, the disease has been virtually eradicated in Canada.
Combination Vaccines
 Diphtheria Toxoid – Pertussis Vaccine (DP), combined, Connaught Laboratories, c.1943
Diphtheria Toxoid – Pertussis Vaccine (DP), combined, Connaught Laboratories, c.1943Image source: Sanofi Pasteur Canada
Canadian scientists, especially at Connaught Laboratories, along with provincial and local public health departments, played a pioneering role in the development and application of combined vaccines, especially for children. The first combination vaccine used in Canada was introduced in 1915, but it was only used in the Army to prevent typhoid and paratyphoid fevers during World War I.
By the early 1940s, the remarkable success of diphtheria toxoid in Canada, coupled with the introduction of a new vaccine to prevent pertussis (whooping cough), created a public health conundrum. More vaccines meant more trips to the doctor for parents and their young kids for another set of shots. In a concerted effort to minimize such trips and immunizations, researchers focused on carefully combining vaccines without affecting the safety and effectiveness of each one in the combination.
 In the U.S., the standard triple combination vaccine was known as DTP. This Park, Davis & Co. DTP package dates from 1956.
In the U.S., the standard triple combination vaccine was known as DTP. This Park, Davis & Co. DTP package dates from 1956.Museum of Health Care, accession #000001472
Initial efforts focused on preparing a combined vaccine that included diphtheria toxoid and pertussis vaccine, DP, which was launched in 1943. After the development of tetanus toxoid in the 1940s, a triple pediatric antigen, DPT, was introduced nationally in 1947, followed by DT as a booster for older children and adults. Broad protection against all three diseases in Canada was thus ensured, particularly as DPT was available for free through local public health clinics or physician’s offices; in the latter case, the physician’s fee was the only cost until the introduction of Medicare in the late 1960s.
The introduction of the Salk polio vaccine in 1955 created a similar challenge of adding more shots. This prompted Connaught to develop the quadruple DPT-Polio vaccine, along with DT-Polio and T-Polio as boosters for older kids and adults, which were launched in early 1959. These combinations consolidated the control of polio in Canada before the introduction of the Sabin oral polio vaccine in 1962, which in several provinces supplanted the Salk vaccine and left the original DPT to be used.
 Toronto Star clipping announcing the launch of DPT-Polio vaccine by Connaught Laboratories, Dec. 15, 1958.
Toronto Star clipping announcing the launch of DPT-Polio vaccine by Connaught Laboratories, Dec. 15, 1958.Image source: Sanofi Pasteur Canada
The next step in the evolution of combination vaccines came in the early 1970s following the introduction of the measles vaccine in the 1960s, followed by a vaccine to prevent rubella (a.k.a German measles) in 1970. A combined measles-rubella vaccine, MR, was first available in 1972, and then a new mumps vaccine was added to create the MMR combination, which came into general use in Canada in 1975.
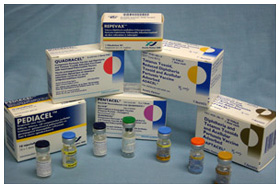
 Family of combination vaccines produced by Sanofi Pasteur, c. 2006.
Family of combination vaccines produced by Sanofi Pasteur, c. 2006.Image source: Sanofi Pasteur Canada
The modern generation of combined vaccines began in the late 1980s with the development of Haemophilus influenza type b, or Hib, vaccine, to prevent a dangerous form of meningitis in young children. Hib was added to DPT-Polio in 1994 to create PENTA. In 1997, Connaught Lab’s pioneering five-component pertussis vaccine became the basis of Sanofi Pasteur Canada’s current family of combination vaccines, such as Pentacel®, Quadracel®, Adacel® and Pediacel®, which vary in the inclusion of Hib and/or Salk polio vaccine.